Table of Contents
- Executive Summary & Key Takeaways for 2025–2029
- Market Size, Growth Forecasts & Revenue Projections
- Latest Technological Advancements in Zygomatic Devices
- Key Manufacturers & Industry Leaders (Official Sources Only)
- Emerging Applications & Expanding Indications
- Regulatory Landscape and Approval Pathways (FDA, CE, etc.)
- Competitive Analysis & Strategic Initiatives
- Regional Trends: High-Growth Markets & Adoption Rates
- Challenges, Barriers, and Future Risks
- Outlook: Opportunities and Game-Changing Innovations to 2029
- Sources & References
Executive Summary & Key Takeaways for 2025–2029
The global landscape for zygomatic orthognathic surgery devices is poised for notable evolution over the 2025–2029 period, driven by advancements in surgical technology, rising demand for facial reconstructive procedures, and a growing emphasis on minimally invasive techniques. Key industry players are investing in innovative solutions that enhance clinical outcomes, reduce operative times, and improve patient recovery experiences.
Among the most significant developments is the integration of digital planning and patient-specific devices. Leading manufacturers such as DePuy Synthes and Zimmer Biomet continue to expand their portfolios of 3D-printed, customized plates and fixation systems designed specifically for complex zygomatic and midface osteotomies. These devices allow for greater surgical precision and tailored anatomical fit, which is especially valuable in managing congenital deformities, trauma, and tumor resection cases.
Data from recent years indicates a steady increase in the incidence of facial trauma and congenital craniofacial conditions globally, thereby fueling the demand for advanced zygomatic surgical interventions. The adoption of resorbable fixation systems, pioneered by companies such as Stryker, is expected to accelerate, given their advantages in pediatric and revision surgeries by eliminating the need for secondary removal procedures.
In 2025 and beyond, the orthognathic devices segment will increasingly embrace digital workflow integration—from preoperative virtual surgical planning to intraoperative navigation systems. This trend is underscored by the development and commercialization of navigation-guided and robotic-assisted platforms, which are now being incorporated into midface procedures by innovators like Brainlab. These technologies promise to elevate accuracy and safety, reducing surgical complications and enhancing patient satisfaction.
Regional analysis suggests North America and Western Europe will continue to lead in adoption due to favorable reimbursement climates and strong hospital infrastructure. However, rapid urbanization, rising healthcare investments, and the expansion of medical tourism are expected to drive above-average market growth in Asia-Pacific and Latin America.
Key takeaways for stakeholders in the zygomatic orthognathic surgery device market for 2025–2029 include:
- Continued shift toward patient-specific, 3D-printed implant and fixation solutions.
- Wider adoption of digital planning tools and intraoperative navigation for improved surgical outcomes.
- Strong pipeline for bioresorbable and minimally invasive device technologies.
- Expanding access and demand in emerging regions, supported by healthcare modernization.
- Heightened competition among established and emerging manufacturers as the market matures.
Overall, the next five years are set to witness accelerated innovation and expanded clinical applications for zygomatic orthognathic surgery devices, with a clear focus on personalization, efficiency, and improved patient care.
Market Size, Growth Forecasts & Revenue Projections
The global market for zygomatic orthognathic surgery devices is poised for moderate but steady growth in 2025 and the subsequent few years, driven by increasing demand for complex maxillofacial procedures, growing awareness of craniofacial deformity corrections, and advancements in surgical device technology. The segment encompasses specialized plates, screws, customized implants, and navigation systems specifically engineered for procedures involving the zygoma (cheekbone) and midface skeletal structures. Among key device manufacturers and suppliers, several established medical technology companies have reinforced their portfolios to address this need, including Smith & Nephew, Zimmer Biomet, Stryker, and DePuy Synthes.
In 2025, the global market size for zygomatic orthognathic surgery devices is estimated to reach several hundred million USD, forming a significant sub-segment of the larger craniomaxillofacial (CMF) devices market. Industry participants are witnessing a compounded annual growth rate (CAGR) in the range of 6-8%, attributed to both an uptick in elective orthognathic surgeries and expanding indications for trauma, tumor resection, and congenital defect repair. The trend is particularly noticeable in North America and Western Europe, where advanced healthcare infrastructure supports greater procedure volumes and adoption of next-generation devices. Moreover, emerging markets in Asia-Pacific are rapidly increasing their share due to healthcare modernization and improved surgical accessibility.
Technological innovation is a prime market driver. Companies such as Stryker and Zimmer Biomet are investing in patient-specific implants and 3D-printed solutions tailored to zygomatic anatomy, enhancing surgical precision and clinical outcomes. The integration of digital planning tools and intraoperative navigation, offered by groups like DePuy Synthes, is expected to bolster the adoption of sophisticated device platforms. As a result, revenue streams for device manufacturers are forecasted to grow not only through direct implant sales but also via value-added services and digital workflow solutions.
Looking ahead into the next few years, the outlook remains optimistic as procedural volumes continue to rise, particularly for reconstructive and aesthetic indications. Key manufacturers are expected to pursue strategic collaborations with surgical centers and invest in surgeon education to further expand their market footprint. Regulatory pathways for novel devices are also likely to become more streamlined, facilitating faster product launches and broader clinical adoption. Overall, the zygomatic orthognathic surgery devices sector is set for continued growth, underpinned by demographic trends, rising procedural sophistication, and ongoing device innovation from leaders such as Smith & Nephew, Stryker, and Zimmer Biomet.
Latest Technological Advancements in Zygomatic Devices
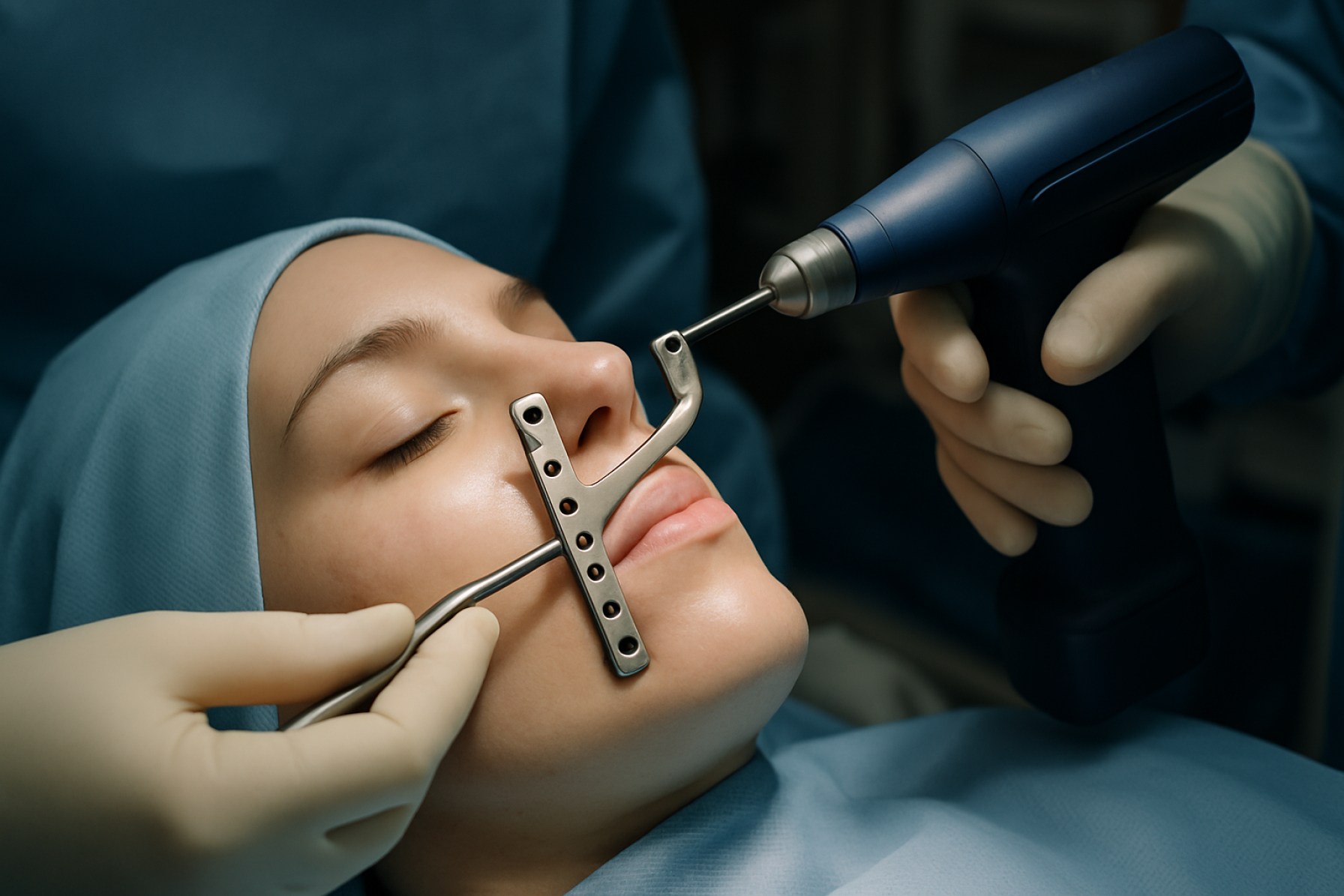
Zygomatic orthognathic surgery devices have witnessed notable technological advancements as of 2025, driven by increasing demand for minimally invasive procedures, improved patient outcomes, and digital integration in surgical planning. One of the most significant trends is the integration of computer-aided design and manufacturing (CAD/CAM) for patient-specific implants and surgical guides. Companies such as Zimmer Biomet and Stryker have expanded their portfolios to include custom zygomatic fixation plates and 3D-printed titanium solutions, enabling more precise anatomical fit and reduced intraoperative time.
Digital workflow advancements now allow for high-resolution 3D imaging, virtual surgical planning, and simulation of osteotomies and implant placements prior to the procedure. These tools facilitate better preoperative visualization and communication among surgical teams. Materialise, for instance, provides software platforms that are widely employed for virtual planning and design of patient-specific surgical instruments in cranio-maxillofacial surgery.
Another major innovation is the evolution of fixation systems. The latest zygomatic plates and screws, often made from biocompatible titanium alloys, are designed for enhanced stability with reduced hardware profile, minimizing patient discomfort and risk of hardware-related complications. Companies like DePuy Synthes have introduced modular fixation systems with low-profile, anatomically contoured plates that are adaptable to various zygomatic complex fracture patterns and osteotomy requirements.
Smart implant technology is emerging, with early prototypes featuring embedded sensors to monitor healing or integration. While not yet mainstream, research collaborations between device manufacturers and academic institutions are expected to yield commercially available smart zygomatic implants within the next few years.
Looking ahead, the focus is shifting toward further automation and intraoperative navigation. Real-time augmented reality (AR) and robotic-assisted surgery platforms are in development, aiming to enhance surgical accuracy and reduce operative risk. Key players such as Medtronic and GE HealthCare are investing in surgical navigation and imaging systems that could be integrated with zygomatic orthognathic procedures.
Overall, the next few years are set to bring further improvements in personalization, efficiency, and outcomes for zygomatic orthognathic surgery, with industry leaders continuing to innovate in device design, digital planning, and operative technologies.
Key Manufacturers & Industry Leaders (Official Sources Only)
The zygomatic orthognathic surgery devices sector is characterized by the presence of several prominent manufacturers and emerging players focused on innovation, precision, and patient-specific solutions. As of 2025, the market is largely driven by advancements in medical device technology, growing emphasis on minimally invasive procedures, and the integration of digital planning and 3D printing for customized implants and fixation systems.
Among the leading manufacturers, DePuy Synthes, a part of Johnson & Johnson, continues to be a key industry player. The company offers a comprehensive portfolio of craniomaxillofacial (CMF) devices, including plates, screws, and patient-specific implants designed for zygomatic and midface reconstruction. Their focus on digital surgical planning and the integration of virtual surgical simulation have positioned them at the forefront of innovation in this field.
Another major manufacturer is Stryker, which provides a wide range of maxillofacial fixation systems, including zygomatic-specific plating solutions. Stryker’s emphasis on research and development and their adoption of additive manufacturing technologies have enabled the production of precise, anatomically contoured devices that cater to individual patient needs.
Europe-based Zimmer Biomet also holds a significant share, offering specialized CMF implants and fixation systems. Their products are widely used in both reconstructive and orthognathic applications, and the company has invested in digital workflow integration to improve surgical outcomes and efficiency.
Emerging companies such as Materialise are making notable contributions through advanced 3D printing services and patient-specific implant solutions. Materialise collaborates with surgeons to design and manufacture custom devices for complex zygomatic procedures, reflecting the shift toward personalized medicine in craniofacial surgery.
Additionally, KLS Martin Group has established itself as a key supplier of CMF surgical devices, including innovative zygomatic fixation products and navigation-assisted surgical systems. Their commitment to surgeon education and support for digital planning tools further strengthens their position.
As the industry advances, manufacturers are increasingly focusing on biocompatible materials, resorbable implants, and enhanced digital planning integration. Partnerships between device companies and technology providers are expected to accelerate the development of next-generation solutions. With ongoing regulatory approvals and growing demand for improved functional and aesthetic outcomes, leading manufacturers are likely to maintain their dominance while fostering opportunities for new entrants with disruptive technologies over the next several years.
Emerging Applications & Expanding Indications
Zygomatic orthognathic surgery devices are witnessing a surge in emerging applications and expanding indications, particularly as surgical techniques and digital planning capabilities evolve. Traditionally, these devices—such as zygomatic plates, screws, and customized fixation systems—were primarily used for correcting severe midfacial deformities, trauma, and congenital anomalies. However, 2025 marks a period of rapid diversification in clinical use, driven by technological advancements and broader acceptance among maxillofacial surgeons worldwide.
One of the most significant trends is the integration of patient-specific implants (PSIs) and computer-aided design/computer-aided manufacturing (CAD/CAM) technology into zygomatic procedures. Companies such as Smith & Nephew and Zimmer Biomet are actively developing and marketing customized zygomatic fixation solutions, enabling precise adaptation to individual patient anatomy and complex craniofacial reconstructions. These tailored devices are increasingly indicated not only for trauma and congenital cases, but also for elective orthognathic procedures addressing facial asymmetry, obstructive sleep apnea (OSA), and age-related midface retrusion.
Another expanding area is the use of zygomatic implants as anchors in dental rehabilitation for patients with severe maxillary atrophy. Companies like Nobel Biocare and Straumann have broadened their portfolios to include devices specifically designed for zygomatic anchorage, reflecting the growing demand from edentulous populations and the trend toward minimally invasive, single-stage rehabilitation protocols.
Recent clinical data and expert consensus point to broader indications for zygomatic devices in orthognathic surgery, including management of syndromic craniofacial conditions, secondary corrections after failed primary surgeries, and oncologic reconstructions. The versatility of modern devices, such as modular plating systems and navigated drilling guides, is facilitating their application in increasingly complex cases, with international surgical societies highlighting expanded guidelines and training initiatives.
Looking ahead, the outlook for zygomatic orthognathic surgery devices is characterized by further indication expansion, especially as 3D printing and digital workflow adoption accelerate. Software-driven planning and intraoperative navigation—supported by industry leaders like DePuy Synthes—are expected to lower complication rates and enable more predictable outcomes in both reconstructive and aesthetic facial procedures. This trajectory, combined with growing patient awareness and surgeon familiarity, points to sustained growth and clinical innovation through 2025 and beyond.
Regulatory Landscape and Approval Pathways (FDA, CE, etc.)
The regulatory landscape for zygomatic orthognathic surgery devices is evolving rapidly as demand grows for advanced maxillofacial surgical solutions. In the United States, these devices are classified as medical devices by the Food and Drug Administration (FDA), generally falling under Class II or Class III, depending on their intended use and risk profile. Manufacturers seeking FDA clearance must typically submit a 510(k) premarket notification demonstrating that their device is substantially equivalent to an already legally marketed device. For more novel or high-risk devices, the more rigorous Premarket Approval (PMA) pathway is required, involving comprehensive clinical data to confirm safety and efficacy. The FDA continues to update guidance for implantable and custom-fabricated devices, reflecting rapid advances in 3D printing and patient-specific implants seen in the zygomatic and craniofacial sector (FDA).
Within the European Union, the Medical Device Regulation (MDR 2017/745) governs the approval and post-market surveillance of zygomatic orthognathic surgery devices. The transition from the former Medical Devices Directive (MDD) to MDR, completed in May 2021, has introduced more stringent requirements for clinical evaluation, risk management, and traceability. Devices must obtain CE marking via a Notified Body, demonstrating conformity with MDR’s essential safety and performance requirements. The MDR emphasizes post-market clinical follow-up and unique device identification—key for tracking patient-specific and custom zygomatic implants. Companies such as Stryker and Zimmer Biomet, both prominent in the craniofacial device space, have adapted their regulatory strategies to align with these regulations, ensuring continued access to the European market.
In other regions, such as Japan and China, approval pathways for zygomatic orthognathic devices are governed by respective national agencies: the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan and the National Medical Products Administration (NMPA) in China. Both agencies have increased scrutiny on imported medical devices, particularly custom and 3D-printed implants, requiring robust clinical and manufacturing evidence.
Looking ahead to 2025 and beyond, the regulatory outlook is characterized by a push for harmonization and digital integration. International bodies, such as the International Medical Device Regulators Forum (IMDRF), continue advancing standards for digital health, software as a medical device (SaMD), and additive manufacturing—developments highly relevant to patient-specific zygomatic implants. Manufacturers like DePuy Synthes and Medtronic are investing in regulatory affairs teams and digital traceability to comply with evolving global requirements. As a result, the market is likely to see faster innovation cycles but also greater regulatory complexity, necessitating close collaboration between device developers, regulators, and clinical partners.
Competitive Analysis & Strategic Initiatives
The competitive landscape for zygomatic orthognathic surgery devices in 2025 is characterized by both established multinational medical device manufacturers and a growing cohort of regional innovators. Key players such as Stryker, Zimmer Biomet, and DePuy Synthes continue to dominate the market through comprehensive product portfolios, robust R&D pipelines, and expansive distribution networks. These companies are investing in advanced plating systems, patient-specific implants, and digital surgical planning tools, aiming to enhance procedural accuracy and reduce operative times.
Strategic initiatives in 2025 reflect a strong emphasis on technological integration and collaboration. For example, Stryker has focused on expanding its craniomaxillofacial (CMF) division, incorporating digital workflow solutions and 3D-printed custom implants to address complex zygomatic reconstructions. Similarly, Zimmer Biomet is leveraging advancements in biomaterials and surface technologies to improve implant osseointegration and patient outcomes. Partnerships between device companies and digital health firms are increasingly common, with several major players announcing collaborations to integrate AI-based surgical planning and intraoperative navigation systems. These alliances aim to streamline preoperative planning and intraoperative guidance, thereby bolstering surgeon confidence and patient safety.
In addition to organic growth, mergers and acquisitions remain a prominent strategy. Major firms have pursued targeted acquisitions of niche technology developers, particularly those specializing in patient-specific solutions and minimally invasive approaches. For instance, acquisitions in the last two years have enabled multinational players to rapidly expand their capabilities in 3D-printing and imaging-guided surgery, positioning themselves at the forefront of personalized maxillofacial interventions.
Meanwhile, regional companies, particularly in Asia-Pacific and Europe, are gaining market share by offering cost-effective alternatives and responding to local clinical needs. Firms such as Medartis have expanded their CMF portfolios with modular plating systems and have increased their presence through direct sales channels and surgeon training programs. These strategies are accompanied by intensified efforts to secure regulatory approvals in new markets, with a focus on compliance and post-market surveillance.
Looking ahead, competitive differentiation will increasingly depend on innovation in digital integration, customization of implants, and enhanced clinical support. Industry leaders are expected to prioritize surgeon education, virtual simulation platforms, and real-world evidence generation to consolidate their market positions. As reimbursement frameworks evolve and demand for complex facial reconstruction grows, the sector is poised for continued investment in R&D and strategic alliances through at least 2027.
Regional Trends: High-Growth Markets & Adoption Rates
In 2025, the zygomatic orthognathic surgery devices market is witnessing significant regional variation in growth and adoption rates, with marked momentum in Asia-Pacific, North America, and select European countries. These trends are shaped by healthcare infrastructure development, rising awareness of advanced maxillofacial procedures, and the expansion strategies of key manufacturers.
Asia-Pacific continues to emerge as a high-growth market, propelled by increasing demand for corrective jaw and facial surgeries in countries such as China, India, and South Korea. This surge is attributed to a combination of rising incidences of congenital craniofacial anomalies, higher rates of trauma cases, and growing preference for minimally invasive and esthetic procedures. Leading device manufacturers such as Zimmer Biomet and Stryker have expanded their distribution networks and localized production facilities in the region to address this growing demand. Moreover, governmental initiatives to improve access to specialized surgical care, coupled with increasing disposable incomes, are further accelerating adoption rates.
In North America, particularly in the United States and Canada, adoption rates remain high due to advanced healthcare infrastructure, a well-established reimbursement system, and the presence of specialised oral and maxillofacial surgery centers. Companies like DePuy Synthes and KLS Martin Group continue to introduce innovative plating systems and patient-specific implants, which are being readily adopted by surgeons in this region. The trend towards digital planning and guided surgical solutions also contributes to the region’s leadership in clinical outcomes and procedural efficiency.
Select European markets—such as Germany, France, and the United Kingdom—are experiencing steady growth, driven by increasing collaborations between hospitals and device manufacturers, as well as the integration of advanced imaging and navigation technologies. The region benefits from a robust network of maxillofacial surgeons and academic centers, assisting in the early adoption of next-generation zygomatic orthognathic devices. European manufacturers, including Medartis, are actively investing in R&D and expanding their regional footprint to meet evolving clinical needs.
Looking ahead, emerging markets in Latin America and the Middle East are expected to register higher adoption rates, supported by improving healthcare access and training initiatives. As global manufacturers continue to localize offerings and develop region-specific solutions, these trends are projected to further accelerate the diffusion of zygomatic orthognathic surgery devices worldwide in the coming years.
Challenges, Barriers, and Future Risks
Zygomatic orthognathic surgery devices, central to advanced maxillofacial reconstruction and correction procedures, face a complex landscape of challenges and risks as the sector moves through 2025 and beyond. While technological progress and surgical demand both rise, several barriers persist that could slow or complicate widespread adoption and innovation.
One of the most significant challenges remains the highly specialized nature of zygomatic procedures, which require precise, anatomically tailored devices such as fixation plates, screws, and custom implants. Manufacturing such devices demands rigorous quality control, stringent regulatory compliance, and ongoing collaboration between oral surgeons and device engineers. Leading manufacturers such as DePuy Synthes and Smith+Nephew must continuously invest in research and development to address the anatomical variability and complexity of the zygomatic region.
Regulatory barriers are also notable. The pathway for approval of medical devices, especially those intended for craniofacial applications, is lengthy and costly. Stringent requirements by bodies like the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) often result in extended timelines for product introduction and can deter smaller manufacturers from entering the market. Compliance with evolving standards—such as those relating to biocompatibility, sterilization, and post-market surveillance—remains a substantial operational burden for device makers.
Another critical barrier is the cost factor. Zygomatic orthognathic surgeries are typically resource-intensive, with devices often custom fabricated or requiring advanced materials like titanium alloys. This drives up procedural costs, which are not always fully reimbursed by insurance providers. Hospitals and surgical centers may hesitate to adopt the latest device innovations if cost-containment pressures persist, potentially slowing clinical adoption even as clinical need rises.
Training and skills development represent ongoing risks. Zygomatic surgeries are technically demanding, and device efficacy is tightly linked to surgical experience and familiarity with specific implant systems. The need for continuous professional education—offered, for example, through programs supported by Zimmer Biomet—is essential but not always universally accessible, particularly in emerging markets.
Looking ahead, cybersecurity and supply chain stability are emerging as new risk factors. As surgical devices increasingly incorporate digital planning, patient-specific design, and even software-enabled functionality, the sector must address risks related to data privacy and cyber-attacks. Furthermore, global events in recent years have highlighted the vulnerability of supply chains for medical-grade metals and precision components, making resilience planning a growing focus for manufacturers such as Stryker.
In summary, while the outlook for zygomatic orthognathic surgery devices is positive due to ongoing innovation and clinical demand, the sector must navigate a multifaceted array of challenges in 2025 and the coming years. Success will depend on technological adaptation, regulatory agility, cost management, and robust practitioner training.
Outlook: Opportunities and Game-Changing Innovations to 2029
The outlook for zygomatic orthognathic surgery devices from 2025 through 2029 is characterized by rapid technological advances, expanding clinical indications, and increased adoption of digital workflows. A major opportunity lies in the integration of 3D planning and patient-specific implant (PSI) technology, which is enabling more precise reconstruction of complex midfacial deformities and trauma. Industry leaders such as Stryker and Zimmer Biomet are investing heavily in digital surgical planning platforms that seamlessly interface with their hardware portfolios, supporting both standard and customized zygomatic fixation solutions.
The uptake of computer-aided design and manufacturing (CAD/CAM) for zygomatic bone plates, meshes, and fixation screws is streamlining operating room workflows and improving surgical accuracy. Custom-milled titanium implants and even bioresorbable alternatives are increasingly available, with companies like KLS Martin Group and DePuy Synthes expanding their offerings of zygomatic-specific osteosynthesis devices. This trend is anticipated to accelerate as more hospitals and surgical teams adopt digital infrastructure and 3D printing capabilities.
Another game-changing innovation set to gain momentum through 2029 is the use of intraoperative navigation and robotics in zygomatic orthognathic surgery. These technologies, championed by device manufacturers such as Medtronic and Brainlab, are improving real-time accuracy in osteotomy placement and reducing intraoperative risk, particularly for complex dysmorphologies and revision procedures. As costs decrease and systems become more user-friendly, their adoption rate is likely to rise, especially in high-volume maxillofacial centers.
The global burden of facial trauma and congenital midface anomalies is expected to drive demand for innovative zygomatic fixation systems, particularly in developing markets where surgical infrastructure is rapidly improving. Companies are responding by launching modular, cost-effective fixation kits tailored to resource-limited settings, while still offering advanced options for high-complexity cases in tertiary care hospitals.
By 2029, ongoing collaboration between device companies, surgical societies, and academic centers is projected to yield further advances in biomaterials, minimally invasive fixation techniques, and digital integration. These developments will continue to reshape the zygomatic orthognathic surgery device sector, promising enhanced outcomes for patients and new opportunities for manufacturers with robust innovation pipelines.
Sources & References
- Zimmer Biomet
- Brainlab
- Smith & Nephew
- Materialise
- Medtronic
- GE HealthCare
- KLS Martin Group
- Nobel Biocare
- Straumann
- Medartis